Abstract
Background
Extramedullary disease (EMD), including central nervous system (CNS) and myeloid sarcoma, is a rare presentation in Acute Myeloid Leukemia (AML). We studied the effect of EMD (CNS involvement only and other EMD ± CNS) compared to isolated BM involvement in the outcomes following hematopoietic stem cell transplantation for AML in children.
Methods
An EBMT registry-based retrospective study to assess the impact of extramedullary disease (EMD) in children (de novo AML, age <18y at transplant, period 2008-2016) with AML who underwent a first allogeneic hematopoietic cell transplant (allo-HCT) with non-TBI conditioning regimen. Outcomes of interest included: leukemia-free survival (LFS), overall survival (OS), relapse incidence (RI) and non-relapse mortality (NRM). Patients were grouped into three categories: BM involvement only (Group A), BM+CNS involvement only (Group B) and BM+ other EMD ± CNS (Group C). Patients with Down syndrome related AML and t(15:17) AML were excluded.
Results
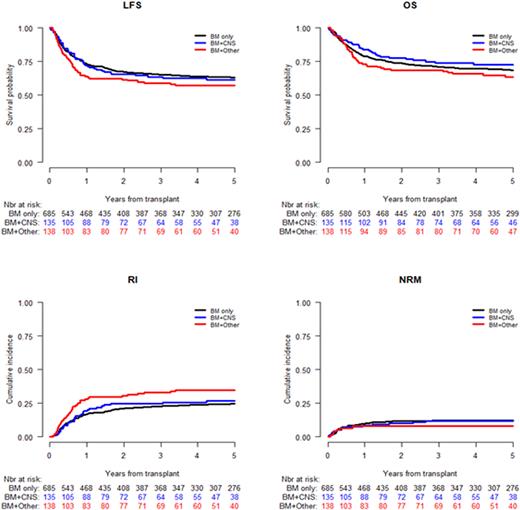
A total of 958 (529 males) patients met study criteria, including 685 (71.5%) Group A, 135 (14.1%) Group B and 138 (14.4%) Group C patients. Group C patients were transplanted at a younger age (median age at transplant 4.8y vs 9.2y and 6.6y for Group A, B respectively, p <0.001). A higher proportion of Group A patients were in CR1 (70.8%) compared to Group B (63.7%) and C (60.1%) patients. The three groups were comparable regarding the distribution of high and standard risk cytogenetics. Fifty seven percent of the patients received an unrelated donor and 35% a matched sibling/family donor. The median post HCT follow-up was 5.4 years (IQR: 5.2-5.7y). Five years LFS, OS, RI and NRM were 62%, 68.4%, 26.4% and 11.6%, respectively in the entire cohort. Multivariate analysis showed higher RI in Group C compared to Group A (HR= 1.45 (1.01-2.06) p=0.04) and a non-significantly different NRM (HR: 0.61 (0.29-1.29), p=0.2). There was no difference in RI in Group B compared to Group A (Hazard Ratio (HR) : 1.12 (0.76-1.64). In the multivariate analysis patients with EMD (CNS only or EMD+/- CNS) had no significant difference in LFS, OS and NRM. The Hazard ratio of LFS, OS and NRM for Group B and C compared to Group A was: HR=1.13, p= 0.46; HR=0.96, p=0.82; HR=1.09, p=0.76 and HR=1.19, p=0.28; HR=0.96, p = 0.82; HR=0.61, p=0.2, respectively.
Further 116 patients underwent hematopoietic stem cell transplantation with active disease. Eighty-nine patients had BM involvement only (group A), 12 patients had BM and CNS disease (Group B) and 15 patients had BM and other sites of EMD +/- CNS (Group C). In this cohort, the 4y-OS was 37% in Group A, 25% in Group B of and 38% in Group C. Four-year LFS was 31%, 25% and 39% in Group A, Groups B and Group C respectively.
Conclusions
Our findings suggest that children with AML and BM with EMD ± CNS involvement (group C) have a higher incidence of relapse after HCT compared to those with BM only or BM+CNS only disease. However, the presence of EMD with CNS involvement or other extramedullary disease did not have a statistically significant impact on overall survival, leukemia-free survival or non-relapse mortality.
Disclosures
Locatelli:Neovii: Speakers Bureau; SOBI: Speakers Bureau; Medac: Speakers Bureau; BlueBird bio: Speakers Bureau; Amgen: Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Miltenyi: Speakers Bureau; Jazz Pharmaceuticals: Honoraria. Bader:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Medac: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Riemser: Research Funding, Speakers Bureau; Neovii: Research Funding; Bristol Myers Squibb: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees; Jazz: Speakers Bureau; Miltenyi: Speakers Bureau. Fagioli:GENZYME SANOFI: Membership on an entity's Board of Directors or advisory committees, Research Funding; JAZZ PHARMA: Membership on an entity's Board of Directors or advisory committees, Research Funding; PFIZER: Membership on an entity's Board of Directors or advisory committees, Research Funding; NOVARTIS: Membership on an entity's Board of Directors or advisory committees, Research Funding; AMGEN: Membership on an entity's Board of Directors or advisory committees, Research Funding; CLINIGEN: Membership on an entity's Board of Directors or advisory committees, Research Funding; TAKEDA: Membership on an entity's Board of Directors or advisory committees, Research Funding. Biffi:Altheia Science: Consultancy. Díaz de Heredia:Biotest: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: meeting and travel expenses ; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: meeting and travel expenses ; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: meeting and travel expenses ; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: meeting and travel expenses .
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal